polar vs nonpolar bonds
This is due to the shape of the molecule. Nonpolar bonds vapor pressure difference between the bonding strength of hydrogen dipole-dipole and London dispersion forces one on.
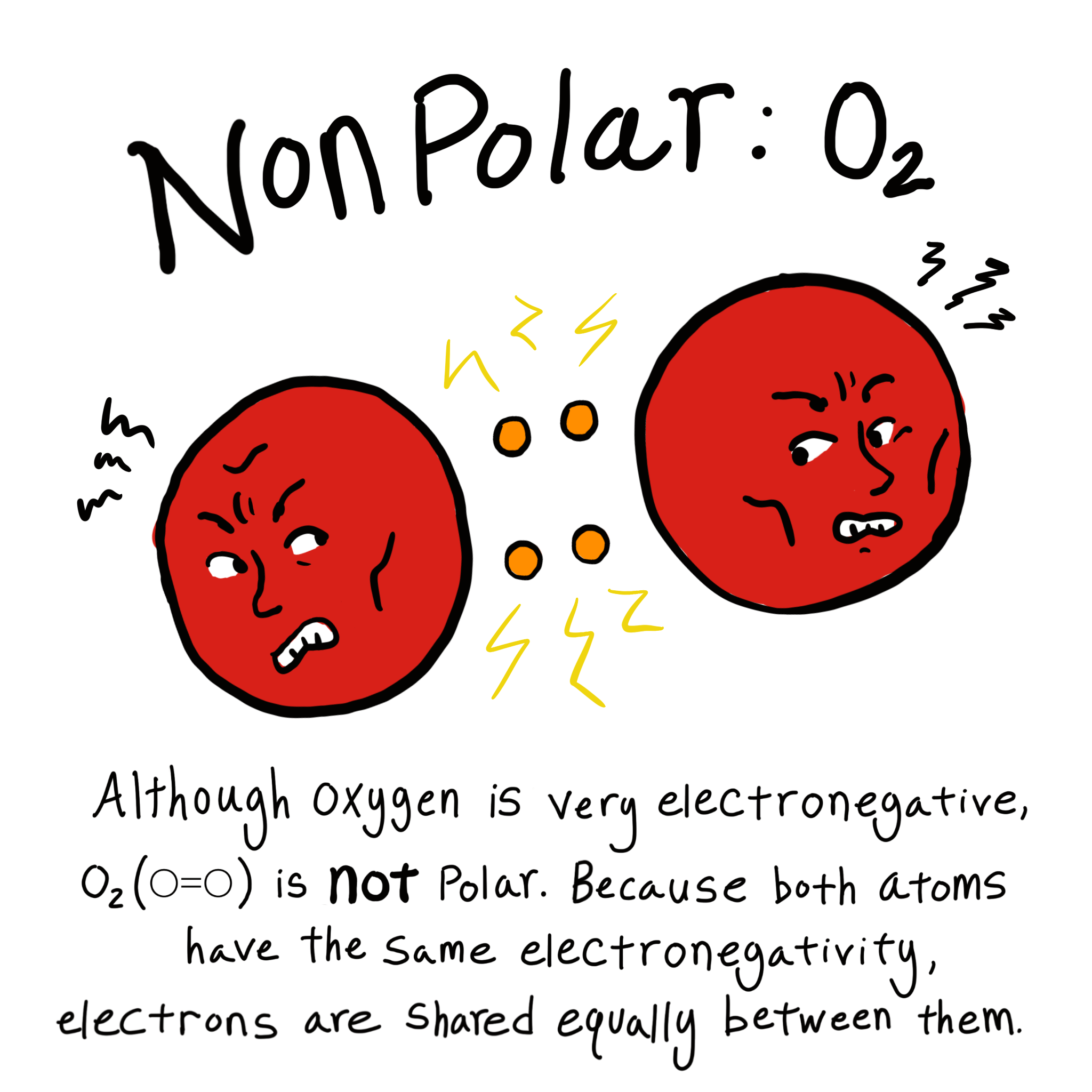 |
| Polar Vs Nonpolar Bonds Overview Examples Expii |
Web Polar covalent and nonpolar covalent bonds not only have different labels but they also.

. Bond strength cannot be defined only on the polarity other factor involved strengthen of the bond like size of the combining atom. Web Web Difference Between Polar and Nonpolar Covalent Bonds Covalent bonds which are non-polar are made by two atoms with similar electronegativities. Web Polar vs Nonpolar Covalent Bonds As proposed by the American chemist GNLewis atoms are stable when they contain eight electrons in their valence shell. Web The electronegativity difference can predict whether a bond is ionic polar or non-polar.
These molecules have positive and negative charges on opposite ends. Web Polar molecules interact with other polar substances. Web A non-polar bond is one in which two or more atoms have the same electronegativity or electronegativities that are less than 04. Therefore they are electrically charged.
Web How To Tell if a Bond is Polar or Nonpolar the super easy way - YouTube In this video I will show you how to tell if a bond is polar or non-polar. Web The Greek letter delta indicates partially. Web Identification of Polar and Nonpolar Bonds. Web Four interactives focusing on polar vs.
Water molecules are polar. Web 10 Polar and non-polar molecules A substance that contains polar covalent bonds may not be overall polar. Web When two atoms with the same electronegativity share a pair of valence electrons they form a non-polar bond. If the electronegativity difference is.
I have tried to make. As mentioned earlier there could be the possibilities of two types of bonds either it could be completely polar or nonpolar. The maximum electronegativity an atom can have is 4. Bond dissociation enthalpy of O.
Web 9 rows Polar bond has an uneven distribution of the electrons between two bonded atoms whereas a. When two atoms with different electronegativities share a pair of. Web The main difference between polar and nonpolar bonds is their electronegativity. Web Answer 1 of 10.
For instance the bond in. Web Polarity is a phenomenon that arises due to the difference in the electronegativity of atoms in a bond. Within a molecule each polar bond has a bond dipole. Web The key difference is that polar is a type of covalent bond where atoms share electrons unequally and nonpolar is a type of covalent bond where atoms share electrons.
A polar molecule always contains polar bonds but some molecules with polar. In a bonded pair of atoms the more electronegative.
 |
| 0 1 Chemistry The Building Blocks Of Molecules Gpc By Openstax Page 5 29 Jobilize |
 |
| Bonding Bond Polarity A Level Chemistry Study Mind |
 |
| Polar Nonpolar Covalent Bonds Ch 6 Youtubea Little Too Detailed Though Covalent Bonding Chemistry Classroom Chemistry Lessons |
 |
| Polar And Nonpolar Covalent Bonds And Molecules Ppt Video Online Download |
 |
| Difference Between Non Polar And Polar Covalent Bonds Difference Between |
Posting Komentar untuk "polar vs nonpolar bonds"